
What’s the connection between this common nasal infection and a leaky gut?
There are many causes of a leaky gut. A MARCoNS nasal infection is one of them. MARCoNS is short for multiple antibiotic resistant coagulase-negative Staphylococci.
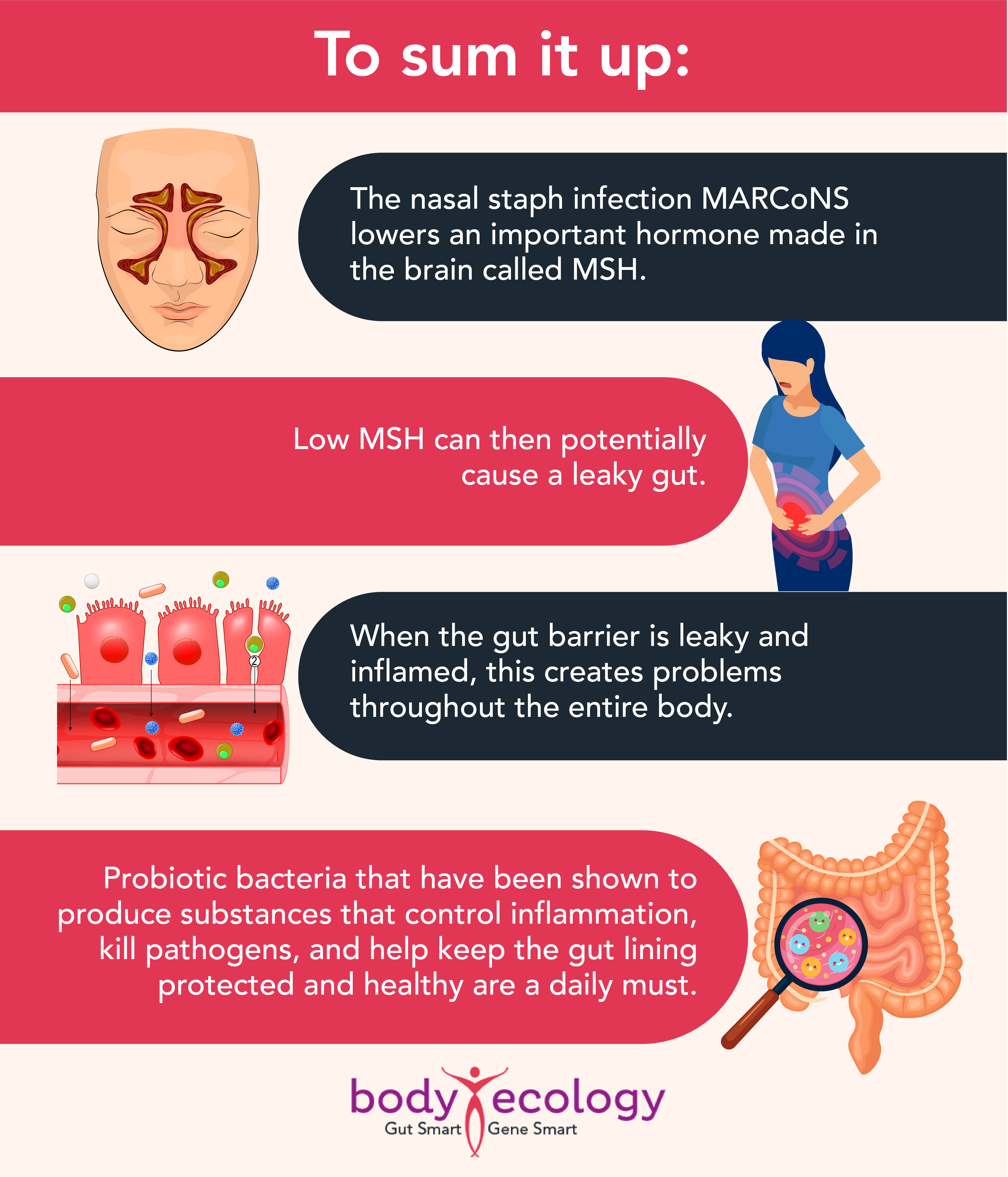
While this staph infection resides in your nasal passages — and is common when people are exposed to mold — MARCoNS can potentially cause a leaky gut by lowering an important hormone called MSH (Melanocyte Stimulating Hormone).
MSH helps regulate other hormones, including cortisol, melatonin, and estrogen. It’s strongly antimicrobial and is part of our innate immune system, also helping to regulate inflammation. You can read more about this here.
Low MSH can cause cortisol to be low, contributing to chronic fatigue. Because adequate levels of cortisol are needed for the thyroid hormone T4 to convert to T3, you may have the symptoms of hypothyroidism. MSH also regulates melatonin, so low MSH may result in non-restful sleep.
The list goes on and on and may include appetite swings, dehydration, gluten intolerance — even though you don’t have celiac disease — and thirst with frequent urination.
And if you have this nasal infection, you’ll most likely have a leaky gut that needs to be treated. A growing body of research suggests that certain probiotics can help heal a leaky gut and support recovery.
For instance, some probiotics may reverse inflammation in the cells that line the intestines.1 They may do this either by directly affecting the function of the intestinal barrier or by competing with and inhibiting the growth and activity of pathogens. This would include MARCoNS and Escherichia coli (E. coli).
Staphylococci are among the first bacterial species to colonize the gut after birth, and the gut is likely to be the primary source for infection with coagulase-negative Staphylococci (CoNS).2,3 This suggests that in the battle against this staph infection, great attention needs to be paid to helping heal the gut in order to prevent reinfection while busting biofilms and eradicating these bacteria.
Here’s a quick lesson in Staph bacteria
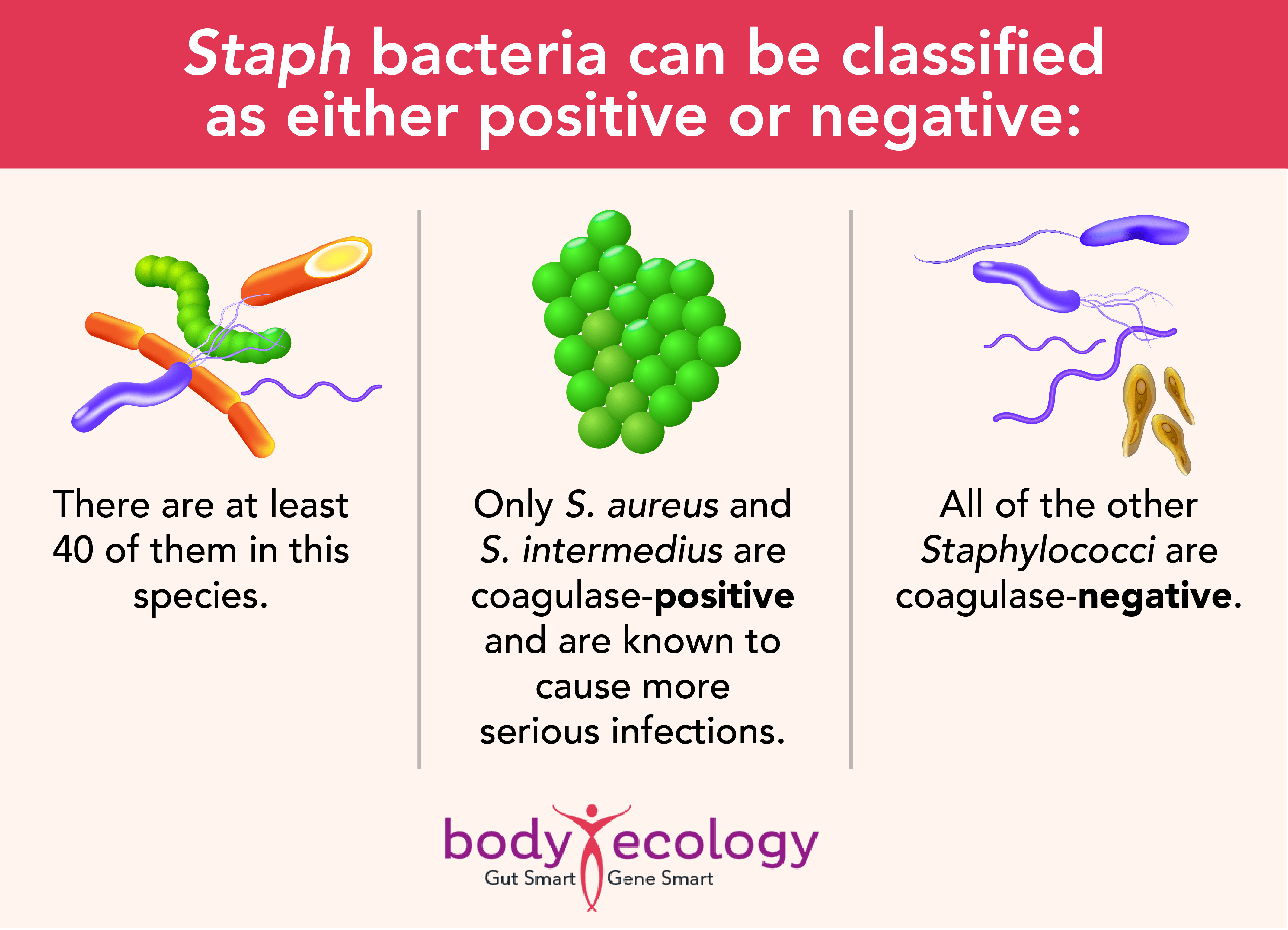
Multiple antibiotic resistance has become increasingly common in S. aureus and S. epidermidis, and resistance to the antibiotic methicillin suggests resistance to multiple antibiotics.
S. epidermidis is the most important CoN species and is the major cause of infections linked to prosthetic devices and catheters, like pacemakers and joint replacements.4 These bacteria may also cause peritonitis in those who receive kidney dialysis and endocarditis (an infection on the lining of the heart) in individuals who have prosthetic valves.5
Other CoNS can cause infections too, but these often go undetected because of difficulties identifying the bacteria. This problem is exacerbated by infections that are often chronic, with few obvious symptoms. While the infection itself may contribute little in the way of active harm, it could help open or keep the door open to other infections by promoting leaky gut syndrome.
Probiotics have been shown to prevent and encourage recovery of leaky gut:
- In one study, a German-made beneficial probiotic E. coli Nissle 1917 (EcN; see Mutaflor.com) was found to prevent intestinal barrier disruption caused by infection with a pathogenic E. coli strain.6
- This probiotic promoted the expression of certain proteins in the tight junction cells in the epithelium, helping to close gaps created by pathogenic bacteria.7,8
Other probiotic species also help support these tight junctions and the epithelium as a whole, in part by secreting metabolites that alter the expression of different proteins in the lining of the gut.
Bifidobacterium infantis, for example, has been found to have beneficial effects on “transepithelial resistance” and “altered ion secretion,” scientific terms that simply mean that the gut is better at keeping in what should be kept in while still letting through beneficial nutrients.9
One of the big hitters in helping to heal a leaky gut, however, is Lactobacillus plantarum:
- The specific strain L. plantarum MB452 has been seen to support gut integrity by affecting occludin and cingulin genes needed for a well-functioning intestinal barrier.10
- When healthy human volunteers took L. plantarum, they had a significant increase in tight junction proteins (ZO-1 and occludin).11
- The researchers in this human study suggest that L. plantarum can enhance the stability of tight junction complexes and may help guard against disruption of the intestinal barrier by cytokines, toxins, and pathogens.
- The probiotic was even seen to protect against chemically-induced disruption of the epithelial barrier in laboratory research.
Lactobacillus casei strain DN-114 001 was found in one study to help “prevent the development of severe forms of intestinal inflammation by strengthening the integrity of intestinal barrier and modulation of gut microenvironment.”12
Lactobacillus rhamnosus GG metabolites were found in another study to help protect against alcohol-induced damage to the intestinal barrier.13
Interestingly, this study didn’t use the live probiotic but instead used the culture in which the bacteria had grown, which was rich with metabolites from the bacteria but not the bacteria themselves. This probiotic supernatant restored levels of tight junction proteins and other factors and helped prevent the typical increase in the intestinal permeability and resulting endotoxemia and liver injury caused by alcohol.
What is candida – and why is it in your body? Claim your free Candida Guide to find out.
Probiotics produce antimicrobial compounds to help control infection
In addition to manufacturing metabolites that support protein production in the intestines, many probiotics produce antimicrobial compounds, including bacteriocins, that can help manage infection directly.14 Lactobacillus salivarius UCC118, for example, produces a bacteriocin (Abp118) that protected mice against infection with Listeria monocytogenes EGDe.15
The bacteriocin Nisin F is produced by Lactococcus lactis subs. lactis and is active against a number of gram-positive bacteria, including Listeria, Staphylococcus, Bacillus, and Clostridium spp.16
In one study, Nisin F was found to temporarily suppress the growth of Staphylococcus aureus in mice.17 In another study, researchers saw an immediate decrease in infection after injection of Nisin F into mice infected with L. monocytogenes EGDe and S. aureus.18
And:
- Bacteriocins, including Nisin, have been shown to have immunomodulatory properties, such as reducing levels of TNF-α and increasing chemokines to help protect against S. aureus, Salmonella enterica sv. Typhimurium, and pathogenic E. coli.19
- Plantaricin 423, produced by L. plantarum 423, is another bacteriocin active against a variety of gram-positive bacteria, including opportunistic pathogens like L. monocytogenes and Enterococcus faecalis.20
- The bacteriocin BacST4SA, produced by E. mundtii ST4SA, is active against L. monocytogenes, S. aureus, and E. faecalis.21
These bacteriocins offer a welcome alternative to antibiotics and could help eradicate a range of bacterial infections, including MARCoNS and other multidrug-resistant infections.22 Bacteriocins are non-toxic to humans and are relatively easy to produce in a laboratory.
Or, of course, you could maintain a healthy diet that includes fermented foods rich in probiotic species producing these bacteriocins.
Probiotics do need to be taken on a daily basis because these tiny little alchemists come and go. Many are merely transient residents inside you; however, while they’re there, they do so many important things.
A spoonful of cultured veggies with your meals will provide you with an amazing diversity of beneficial bacteria and other microbes. But introducing specific strains of other potent bacteria — like Bifidus and Bacillus species — make for an even healthier gut.
REFERENCES:
- 1. Resta-Lenert S, Barrett KE. Probiotics and commensals reverse TNF-alpha- and IFN-gamma-induced dysfunction in human intestinal epithelial cells. Gastroenterology. 2006 Mar;130(3):731-46. doi: 10.1053/j.gastro.2005.12.015. PMID: 16530515.
- 2. Adlerberth I, Lindberg E, Aberg N, Hesselmar B, Saalman R, Strannegård IL, Wold AE. Reduced enterobacterial and increased staphylococcal colonization of the infantile bowel: an effect of hygienic lifestyle? Pediatr Res. 2006 Jan;59(1):96-101. doi: 10.1203/01.pdr.0000191137.12774.b2. Epub 2005 Nov 30. PMID: 16380405.
- 3. Wade JC, Schimpff SC, Newman KA, Wiernik PH. Staphylococcus epidermidis: an increasing cause of infection in patients with granulocytopenia. Ann Intern Med. 1982 Oct;97(4):503-8. doi: 10.7326/0003-4819-97-4-503. PMID: 7125409.
- 4. James G.H. Dinulos, Nicole C. Pace, Chapter 12 – Bacterial Infections, Editor(s): Lawrence F. Eichenfield, Ilona J. Frieden, Nancy B. Esterly, Neonatal Dermatology (Second Edition), W.B. Saunders, 2008, Pages 173-191, ISBN 9781416034322, https://doi.org/10.1016/B978-1-4160-3432-2.50015-7.
- 5. Foster T. Staphylococcus. In: Baron S, editor. Medical Microbiology. 4th edition. Galveston (TX): University of Texas Medical Branch at Galveston; 1996. Chapter 12.
- 6. Zyrek AA, Cichon C, Helms S, Enders C, Sonnenborn U, Schmidt MA. Molecular mechanisms underlying the probiotic effects of Escherichia coli Nissle 1917 involve ZO-2 and PKCzeta redistribution resulting in tight junction and epithelial barrier repair. Cell Microbiol. 2007 Mar;9(3):804-16. doi: 10.1111/j.1462-5822.2006.00836.x. Epub 2006 Nov 3. PMID: 17087734.
- 7. Veltman K, Hummel S, Cichon C, Sonnenborn U, Schmidt MA. Identification of specific miRNAs targeting proteins of the apical junctional complex that simulate the probiotic effect of E. coli Nissle 1917 on T84 epithelial cells. Int J Biochem Cell Biol. 2012 Feb;44(2):341-9. doi: 10.1016/j.biocel.2011.11.006. Epub 2011 Nov 15. PMID: 22101077.
- 8. Ukena SN, Singh A, Dringenberg U, et al. Probiotic Escherichia coli Nissle 1917 inhibits leaky gut by enhancing mucosal integrity. PLoS One. 2007;2(12):e1308. Published 2007 Dec 12. doi:10.1371/journal.pone.0001308.
- 9. Corridoni D, Pastorelli L, Mattioli B, et al. Probiotic bacteria regulate intestinal epithelial permeability in experimental ileitis by a TNF-dependent mechanism. PLoS One. 2012;7(7):e42067. doi:10.1371/journal.pone.0042067.
- 10. Anderson RC, Cookson AL, McNabb WC, et al. Lactobacillus plantarum MB452 enhances the function of the intestinal barrier by increasing the expression levels of genes involved in tight junction formation. BMC Microbiol. 2010;10:316. Published 2010 Dec 9. doi:10.1186/1471-2180-10-316.
- 11. Karczewski J, Troost FJ, Konings I, Dekker J, Kleerebezem M, Brummer RJ, Wells JM. Regulation of human epithelial tight junction proteins by Lactobacillus plantarum in vivo and protective effects on the epithelial barrier. Am J Physiol Gastrointest Liver Physiol. 2010 Jun;298(6):G851-9. doi: 10.1152/ajpgi.00327.2009. Epub 2010 Mar 11. PMID: 20224007.
- 12. Zakostelska Z, Kverka M, Klimesova K, et al. Lysate of probiotic Lactobacillus casei DN-114 001 ameliorates colitis by strengthening the gut barrier function and changing the gut microenvironment. PLoS One. 2011;6(11):e27961. doi:10.1371/journal.pone.0027961.
- 13. Wang Y, Liu Y, Sidhu A, Ma Z, McClain C, Feng W. Lactobacillus rhamnosus GG culture supernatant ameliorates acute alcohol-induced intestinal permeability and liver injury. Am J Physiol Gastrointest Liver Physiol. 2012;303(1):G32-G41. doi:10.1152/ajpgi.00024.2012.
- 14. Dobson A, Cotter PD, Ross RP, Hill C. Bacteriocin production: a probiotic trait?. Appl Environ Microbiol. 2012;78(1):1-6. doi:10.1128/AEM.05576-11.
- 15. Corr SC, Li Y, Riedel CU, O’Toole PW, Hill C, Gahan CG. Bacteriocin production as a mechanism for the antiinfective activity of Lactobacillus salivarius UCC118. Proc Natl Acad Sci U S A. 2007;104(18):7617-7621. doi:10.1073/pnas.0700440104.
- 16. Dreyer L, Smith C, Deane SM, Dicks LMT, van Staden AD. Migration of Bacteriocins Across Gastrointestinal Epithelial and Vascular Endothelial Cells, as Determined Using In Vitro Simulations. Sci Rep. 2019;9(1):11481. Published 2019 Aug 7. doi:10.1038/s41598-019-47843-9.
- 17. Brand AM, de Kwaadsteniet M, Dicks LM. The ability of nisin F to control Staphylococcus aureus infection in the peritoneal cavity, as studied in mice. Lett Appl Microbiol. 2010 Dec;51(6):645-9. doi: 10.1111/j.1472-765X.2010.02948.x. Epub 2010 Oct 11. PMID: 21029139.
- 18. Brand AM, Smith R, de Kwaadsteniet M, Dicks LM. Development of a Murine Model with Optimal Routes for Bacterial Infection and Treatment, as Determined with Bioluminescent Imaging in C57BL/6 Mice. Probiotics Antimicrob Proteins. 2011 Jun;3(2):125-31. doi: 10.1007/s12602-011-9069-4. PMID: 26781578.
- 19. Kindrachuk J, Jenssen H, Elliott M, Nijnik A, Magrangeas-Janot L, Pasupuleti M, Thorson L, Ma S, Easton DM, Bains M, Finlay B, Breukink EJ, Georg-Sahl H, Hancock RE. Manipulation of innate immunity by a bacterial secreted peptide: lantibiotic nisin Z is selectively immunomodulatory. Innate Immun. 2013 Jun;19(3):315-27. doi: 10.1177/1753425912461456. Epub 2012 Oct 29. PMID: 23109507.
- 20. Botes M, Loos B, van Reenen CA, Dicks LM. Adhesion of the probiotic strains Enterococcus mundtii ST4SA and Lactobacillus plantarum 423 to Caco-2 cells under conditions simulating the intestinal tract, and in the presence of antibiotics and anti-inflammatory medicaments. Arch Microbiol. 2008 Nov;190(5):573-84. doi: 10.1007/s00203-008-0408-0. Epub 2008 Jul 19. PMID: 18641972.
- 21. Botes M, van Reenen CA, Dicks LM. Evaluation of Enterococcus mundtii ST4SA and Lactobacillus plantarum 423 as probiotics by using a gastro-intestinal model with infant milk formulations as substrate. Int J Food Microbiol. 2008 Dec 10;128(2):362-70. doi: 10.1016/j.ijfoodmicro.2008.09.016. Epub 2008 Oct 1. PMID: 18963159.
- 22. Daniel Walker, Hannah M. Behrens, Anne Six, Daniel Walker, Colin Kleanthous; The therapeutic potential of bacteriocins as protein antibiotics. Emerg Top Life Sci 21 April 2017; 1 (1): 65–74. doi: https://doi.org/10.1042/ETLS20160016.









